Promotional information from
Prescribing information and adverse event reporting:
ASEAN | Brazil | Germany | Italy | Korea | Kuwait-Lebanon | Netherlands | Spain | Switzerland | UAE
Headache Treatment Hub
The body of evidence for the use of Emgality®▼ (galcanezumab)** as a preventive migraine medication is increasing, as shown in several congresses throughout 2020.

The nature of interictal burden in migraine and the effect of galcanezumab on interictal burden
Take-home messages:
- The interictal burden migraine imposes on patients is substantial1
- Galcanezumab reduced both the ictal and interictal burden of migraine in patients with treatment-resistant migraine2
- Multiple outcomes should be used in measuring the burden of migraine and the efficacy of preventive treatments for migraine2
Based on:
Lipton R B, Buse D C et al. Interictal burden of migraine: correlations with other measures of migraine burden and effects of galcanezumab migraine-preventive treatment. Presented at 14th European Headache Federation (EHF), Virtual 2020, 29 June-2 July 2020 (Presented by Richard Lipton). Available from: lillyscience.lilly.com. Accessed May 2021. Poster #272.
Objective and background
Migraine imposes a substantial interictal burden on patients; as many as 76% of migraineurs worry that they will have migraines for the rest of their lives, and 37% worry about migraines between attacks. Anticipation of future migraine attacks can cause anxiety, decreased feelings of well-being and disturbed sleep, and patients may restrict their activities due to concern of future attacks.1
In this analysis of the CONQUER study, presented by Richard Lipton, Albert Einstein College of Medicine of Yeshiva University, Bronx, NY, US, the aim was to explore the nature of interictal burden in migraine, and to determine if treatment with galcanezumab could reduce this burden.2
CONQUER trial design and interictal burden correlation analysis
CONQUER was a phase IIIb, multicentre, double-blind, placebo-controlled trial, assessing the efficacy and tolerability of galcanezumab in patients with episodic or chronic migraine. Patients were randomised to monthly treatment with placebo (n=230) or galcanezumab 120 mg (n=232). Key inclusion criterion was for patients to have failed 2-4 prior migraine preventive treatment categories in the past 10 years due to to inadequate efficacy and/or safety/ tolerability reasons.2,3
Patient-reported outcomes and outcome measurements of the study that were included in the correlation analysis were:2
| Patient-reported outcomes | Outcome measurement | |
| MIBS-4* | Migraine Interictal Burden Scale | Disruptions to life on days without headache |
| MIDAS | Migraine Disability Assessment | Disability due to migraine headache |
| MSQ v2.1 | Migraine-Specific Quality of Life Questionnaire | Functioning in various aspects of life |
| PGI-S | Patient Global Impression of Severity | Severity of overall migraine illness |
| GAD-7 | Generalised Anxiety Disorder | Anxiety symptoms |
| PHQ-9 | Patient Health Questionnaire | Depression symptoms |
| WPAI | Work Productivity and Activity Impairment | Impact of migraine on ability to do work and regular activities |
| Additional outcomes | Outcome measurement | |
| Monthly migraine headache days | Days per month with migraine headache | |
| Symptom-free headache-free days | Days per month with no headache or other migraine symptoms | |
*The level of interictal burden was classified as ‘none’, ‘mild’, ‘moderate’ or ‘severe’ for MIBS-4 scores of 0, 1-2, 3-4, or 5-12, respectively. MIBS-4 score was determined using the migraine interictal burden survey.1,2
Correlations between outcomes were determined using the non-parametric Spearman’s rank correlation co-efficient (rho) because it was assumed the data were not normally distributed. The degree of correlation was interpreted as ‘very high’ (absolute value of rho: >0.90 to ≤1.00), ‘high’ (>0.70 to ≤0.90), ‘moderate’ (>0.50 to ≤0.70), ‘low’ (>0.30 to ≤0.50) or ‘negligible’ (0.00 to ≤0.30).2,4
Result:
The treatment with galcanezumab was associated with a greater reductions versus placebo in the ictal as well as interictal burden of migraine, the latter measured by MIBS-4.2

†p<0.0001 vs PBO
GMB: galcanezumab; LS: least squares; PBO: placebo; SE: standard error.
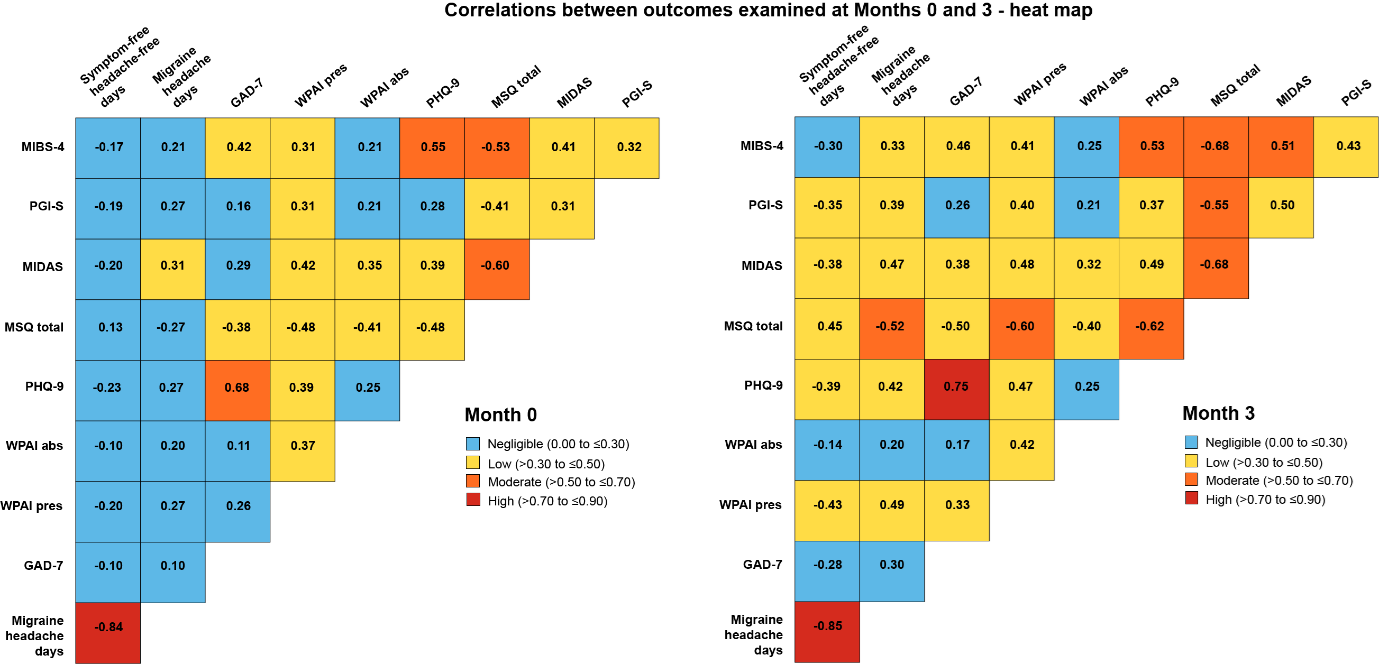
The correlations for MIBS-4 scores for all patients (placebo and galcanezumab) with other outcomes were negligible to moderate:2
- The highest MIBS-4 correlations were with MSQ total and PHQ-9, which both moderately correlated with MIBS-4 at Months 0 and 3
- For most outcomes, the level of correlation with MIBS-4 was consistent for Months 0 and 3, though there was a trend of increased value for rho at Month 3

The analysis also assessed the correlations between several other outcomes at Months 0 and 32
- Most correlations were of negligible to low strength and only two correlations were high
- Symptom- or headache-free days had negligible to low correlations with all patient-reported outcomes at Months 0 and 3, and high correlation with migraine headache days
- Few moderate and no high correlations were observed for patient-reported outcomes at Month 0:
- PHQ-9 with GAD-7
- MIBS-4 with PHQ-9 and MSQ total
- MSQ total with MIDAS
- Five correlations transitioned from negligible/ low at Month 0 to moderate at Month 3:
- MIBS-4 with MIDAS
- MSQ total with PGI-S, WPAI presenteeism, PHQ-9 and migraine headache days
Conclusion2
In addition to reducing ictal burden, treatment with galcanezumab was associated with reductions in the interictal burden of migraine as measured by MIBS-4. The correlation of the MIBS-4 and monthly migraine headache days was negligible to low at 0.21-0.33, indicating that ictal measures, such as migraine headache days, do not fully capture the interictal burden in migraine. MIBS-4 most strongly correlated with PHQ-9 and MSQ total. Since the MSQ was designed to measure both ictal and interictal burden of migraine, this correlation would be expected. The results show that multiple outcomes should be measured when evaluating the efficacy of preventive treatments for migraine to better understand their implications for patients.
**Emgality (galcanezumab) is indicated for the prophylaxis of migraine in adults who have at least 4 migraine days per month.5
References
- Buse D C, Rupnow M F T, Lipton R B. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clinic proceedings 2009;84(5):422-435
- Lipton R B, Buse D C et al. Interictal burden of migraine: correlations with other measures of migraine burden and effects of galcanezumab migraine-preventive treatment. Presented at 14th European Headache Federation (EHF), Virtual 2020, 29 June-2 July 2020. Available from: lillyscience.lilly.com. Accessed May 2021. Poster #272.
- Mulleners W M, Kim B K et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 2020;19(10):814-825
- Mukaka M M. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012;24(3):69-71
- Emgality: Brazilian label approved by Anvisa. Available from: consultas.anvisa.gov.br. Accessed May 2021
You have to report Lilly product related adverse events (AE) / product complaints (PC) as required by local laws and regulations in your country. For any AE/PC occurring for a Lilly commercialised product, please contact your local Lilly country representative.
CDS29ABR19
EMGALITY® (galcanezumabe) Indicações: EMGALITY é indicado para a profilaxia da enxaqueca em adultos que apresentam pelo menos quatro dias de enxaqueca por mês. EMGALITY é indicado para a prevenção de crises durante o período de salvas em adultos com cefaleia em salvas episódica. Contraindicações: EMGALITY é contraindicado para pacientes com hipersensibilidade grave conhecida a galcanezumabe ou a qualquer um de seus excipientes. Advertências e Precauções: reações graves de hipersensibilidades foram relatadas. Se ocorrer uma reação grave de hipersensibilidade, descontinuar EMGALITY imediatamente e iniciar a terapia apropriada. Do mesmo modo que com todas as proteínas terapêuticas, existe o potencial de imunogenicidade com EMGALITY. Pacientes com certas doenças cardiovasculares graves foram excluídos dos estudos clínicos de cefaleia em salvas episódica, portanto, não há dados de segurança disponíveis nesses pacientes. Há dados insuficientes em humanos para estabelecer a segurança de EMGALITY durante a gestação. EMGALITY deve ser utilizado na gestação somente se o benefício potencial justificar o possível risco à mãe ou ao feto. Os benefícios da amamentação para o desenvolvimento e a saúde devem ser considerados, junto à necessidade clínica da mãe por EMGALITY e qualquer potencial efeito adverso no bebê amamentado. EMGALITY pode ter uma pequena influência sobre a capacidade de conduzir e utilizar máquinas. Vertigem pode ocorrer após a administração de EMGALITY. A segurança e a eficácia de EMGALITY não foram estabelecidas em pacientes pediátricos menores de 18 anos de idade. Enxaqueca: há informações limitadas em pacientes geriátricos maiores de 65 anos de idade. Cefaleia em salvas: os estudos clínicos de galcanezumabe não incluíram número suficiente de pacientes com 65 anos ou mais para determinar se eles respondem diferentemente de pacientes mais jovens. Interações medicamentosas: interações medicamentosas farmacocinéticas não são esperadas, com base nas características de EMGALITY. Nenhum estudo foi conduzido para investigar possível interação entre EMGALITY e plantas medicinais, álcool, nicotina e exames laboratoriais e não laboratoriais. Posologia e modo de usar: EMGALITY é para administração subcutânea. Enxaqueca: a dose recomendada é de 120 mg, injetada pela via subcutânea uma vez por mês, com uma dose de ataque de 240 mg como dose inicial. O benefício do tratamento deve ser avaliado em até 3 meses após o seu início. A decisão de continuar o tratamento deverá ser tomada individualmente para cada paciente. Posteriormente, é recomendada a avaliação da necessidade de continuar o tratamento regularmente. Cefaleia em salvas: a dose recomendada é de 300 mg (três injeções subcutâneas consecutivas de 100 mg cada), injetada pela via subcutânea uma vez por mês, durante o período de salvas. Reações adversas: dados de estudos clínicos - muito comum ≥ 10%: dor no local da injeção e reações no local da injeção (excluindo dor); comum (≥ 1% e < 10%): vertigem, constipação e prurido. Dados pós-comercialização - comum (≥ 1% e < 10%): rash. Venda sob prescrição médica. Registro MS - 1.1260.0200. Documentação científica e/ou informações adicionais à classe médica sobre o produto mediante solicitação. Para mais informações, consulte a bula completa do produto ou o Serviço de Atendimento ao Cliente Lilly SAC 0800 701 0444, e-mail: sac_brasil@lilly.com. 13/05/2020, 06/01/2020. Contraindicações: EMGALITY é contraindicado para pacientes com hipersensibilidade grave conhecida a galcanezumabe ou a qualquer um de seus excipientes. Interações medicamentosas: interações medicamentosas farmacocinéticas não são esperadas, com base nas características de EMGALITY. Nenhum estudo foi conduzido para investigar possível interação entre EMGALITY e plantas medicinais, álcool, nicotina e exames laboratoriais e não laboratoriais.
Material destinado exclusivamente a profissionais de saúde habilitados a prescrever e dispensar medicamentos.